PMV Pipeline
PMV Pharma is at the forefront of precision oncology, focusing on the discovery and development of small-molecule therapies. Our tumor agnostic approach targets specific genetic alterations, developing therapies not restricted to a specific cancer or tissue type. Rezatapopt (PC14586), a first-in-class selective p53 reactivator, targeting p53 Y220C mutation, is currently undergoing clinical trials for patients with advanced solid tumors alone or in combination with pembrolizumab. We are exploring additional undisclosed targets, including other p53 hotspot mutations, which hold promise in treating various tumors, advancing precision medicine and improving patient outcomes.
rezatapopt and azacitidine (IIT*)
Target:p53 mutation Y220C
Indication:Relapsed/Refractory AML/MDS
Undisclosed
Target:Undisclosed
Indication:Solid Tumors
1 In Discovery, we screen compounds against biological assays to identify lead compounds.
2 In Lead Optimization, we modify the lead compound to improve potency, selectivity, pharmacokinetic and toxicity parameters and physical chemical properties important for clinical development.
3 In IND-Enabling Studies, we conduct preclinical studies, in accordance with Good Laboratory Practice, or GLP, required for an IND submission to the FDA.
* Investigator Initiated Trial.
rezatapopt (PC14586)
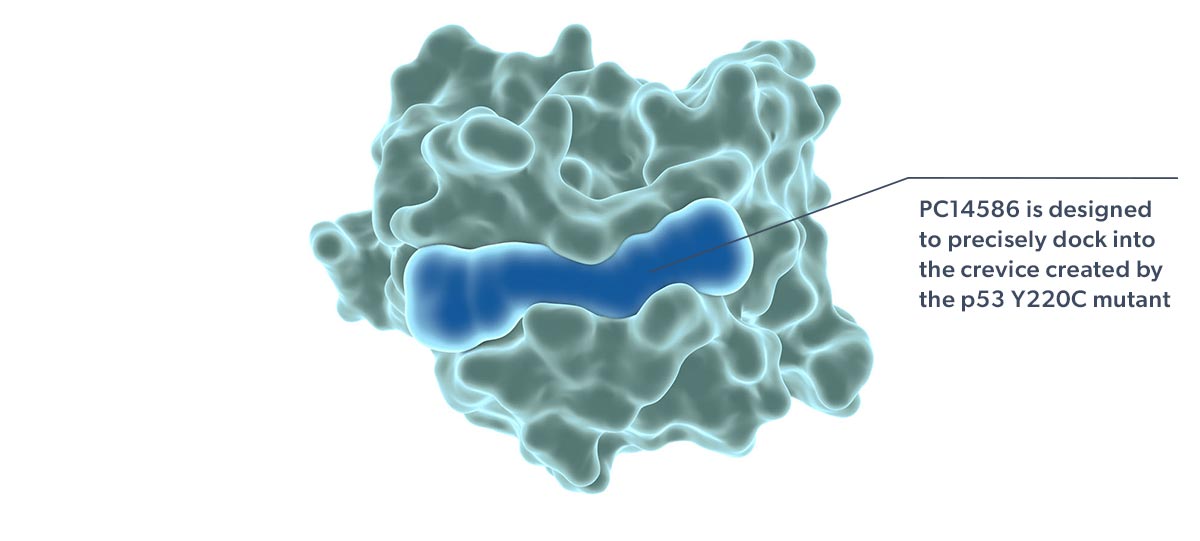
Our lead product candidate, rezatapopt, is an orally available small molecule for the treatment of patients with locally advanced or metastatic solid tumors that have a p53 Y220C mutation. Rezatapopt is an investigational product and has not yet been approved by the US Food and Drug Administration (FDA) or any regulatory authority.
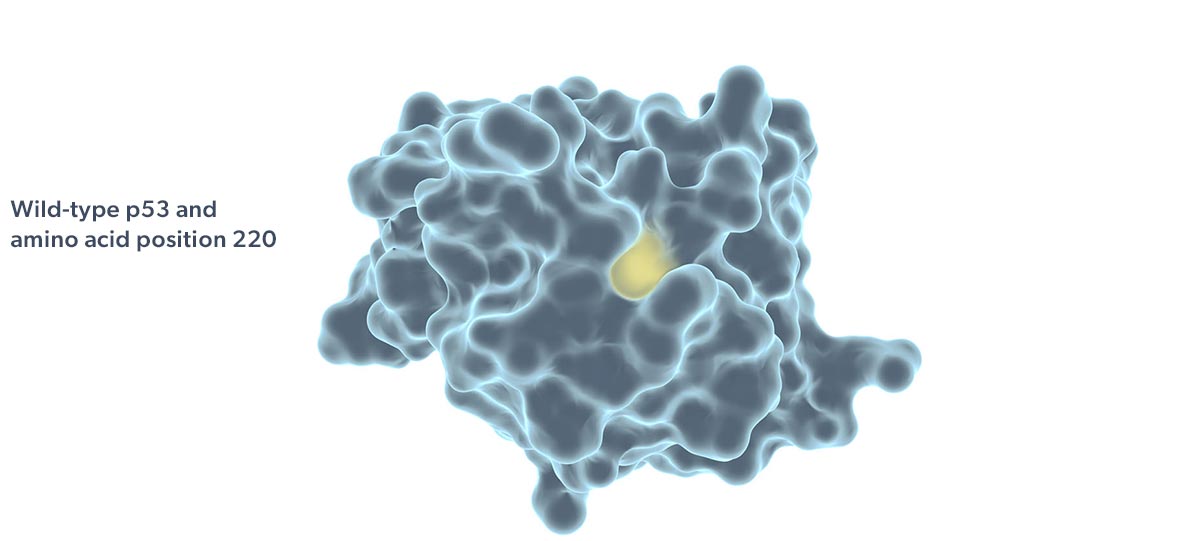
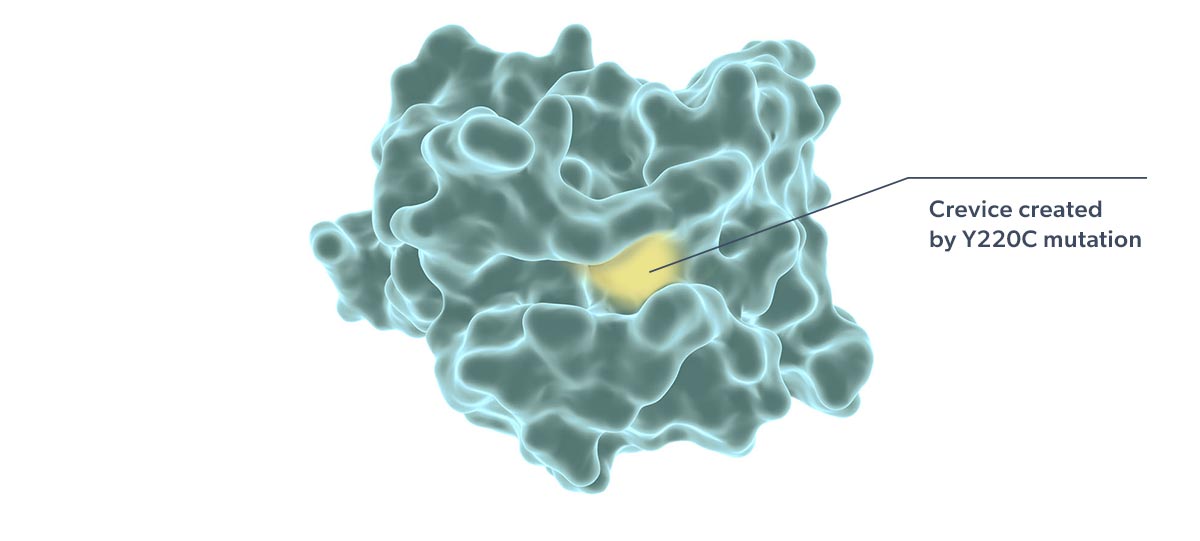
Rezatapopt, a first-in-class selective p53 reactivator, is designed to stabilize the p53 Y220C mutant in which tyrosine is substituted by cysteine at amino acid 220 of the protein. This single amino acid change creates a small crevice in the p53 protein, making it thermally unstable and unable to effectively interact with DNA. Targeting this crevice with a small molecule to restore function to p53 Y220C has been proposed as a potential therapeutic strategy (Joerger 2006; Basse 2010).
The p53 Y220C mutation is the ninth most frequent p53 mutation and accounts for 1.8% of all p53 mutations (Bouaoun 2016). The p53 Y220C mutation is observed in at least 30 different tumor types.
The US FDA has granted Fast Track designation to rezatapopt for the treatment of cancer patients with locally advanced or metastatic solid tumors that have a p53 Y220C mutation.
PMV-Sponsored Clinical Studies
PMV-586-101
A Phase 1/2 Open-label, Multicenter Study to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of rezatapopt (PC14586) in Patients with Advanced Solid Tumors Harboring a p53 Y220C Mutation.
To learn more about this clinical study please visit www.clinicaltrials.gov, study identifier NCT04585750 , or www.PYNNACLEstudy.com.

