p53 Overview
Tumor suppressor p53: guardian of the genome
p53 is profoundly important in human biology. p53 plays a pivotal role in cellular function by preserving the integrity of DNA and preventing abnormal cells from entering or progressing through the cell cycle (Blandino 2019; Levine 2019). This is why p53 is referred to as the guardian of the genome (Lane 1992).
Damaged DNA and cellular stress during cell replication can result in mutations that cause cancer. Situated at the nexus of multiple regulatory pathways, p53 continuously monitors the cell for DNA damage and cellular stress. Activated p53 will bind to the DNA site, determining the fate of the cell by activating downstream genes that lead to cell cycle arrest, cell senescence, or apoptosis.
p53 and Cancer
p53 mutation and onset of oncogenic actions
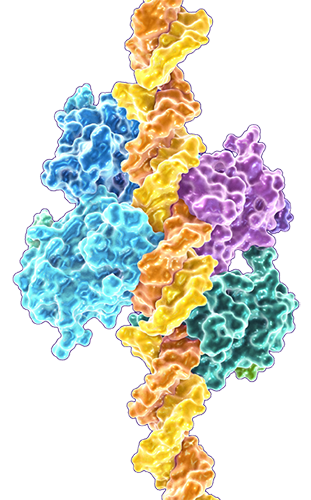
The function of wild-type (normal) p53 is linked to its complex shape and flexibility of DNA-binding domains on the protein.
A single alteration in the amino acid sequence of the p53 protein can affect its ability to bind to DNA and suppress tumor growth. Most of these mutations occur in the central DNA binding domain and are referred to as hot-spot mutations (Baugh 2018; Toufektchan 2018).
Mutated p53 takes on oncogenic properties that endow cancer cells with a growth advantage and resistance to anti-cancer therapy (Hong 2014).

wild-type (normal) p53
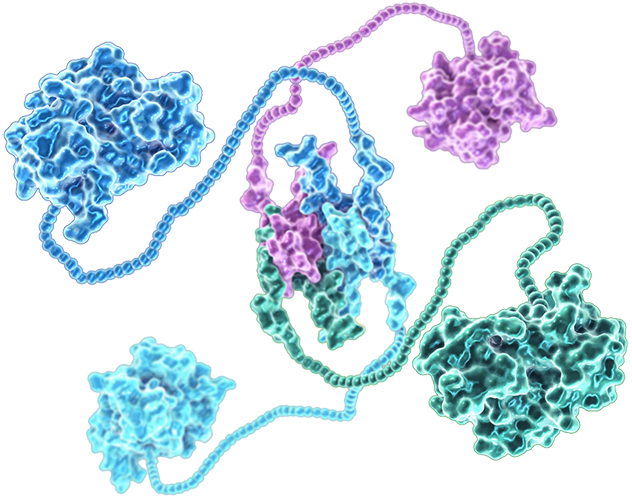
Mutated p53 unfolds and
loses its DNA binding ability
One of the consequences of p53 mutations and disruption to p53 regulatory pathways is tumor formation and growth. p53 mutations are found in approximately half of all human cancers including breast, lung, prostate, colorectal, and uterine cancers.
Drug Discovery
Developing customized tumor-agnostic therapeutics to restore wild-type p53
PMV Pharma is pioneering the discovery and development of small molecule therapeutics that selectively target mutant p53. We have leveraged more than four decades of research experience and developed unique insights into p53 since its discovery in 1979 by PMV co-founder, Dr. Arnold Levine. One of the biggest challenges in moving from discovery to therapeutic application has been the complexity of p53 and the extraordinary diversity of p53 mutations in the DNA-binding domain of the protein (Levine 2019).
PMV Pharma takes a unique approach to restoring mutant p53 by physically correcting the precise geometry of the protein, thereby reactivating its tumor suppressor function. We are leveraging our precision oncology platform to design and develop a pipeline of selective, small molecule, tumor-agnostic therapies that structurally correct specific mutant p53 proteins to restore their wild-type function.

